How do side branches in acute Type-B aortic dissections affect stabilization of the arterial wall? - Insights from an idealized fluid-solid interaction study
- Jul 28, 2025
- 4 min read
By: Amith Balasubramanya, Lise Gheysen, Markus U Wagenhauser, Nele Famaey, Joris Degroote, Patrick Segers

Aortic dissections are life–threatening conditions in which one or more tears occur in the inner layer of the aorta – the body’s main artery. These tears allow for an exchange of blood between the original true lumen of the aorta and an alternative channel alongside but within the aortic wall called the false lumen. Both are separated by a thin, flexible membrane, the intimal flap. Clinicians classify dissections based on their location, with Type-B dissections presenting in the part of the aorta running down from the aortic arch towards the abdomen. During the first two weeks after the dissection starts—known as the acute phase—the intimal flap is thin and highly mobile. The interaction between the mobile flap (solid) and surrounding blood (fluid) leads to complex flow patterns and is likely to affect blood pressure and other flow metrics, which makes this a complex Fluid-Solid interaction (FSI) problem.
Side branches of the aorta, emanating from the false lumen, are often overlooked in medical imaging and computer simulations because of their small size and difficulty in imaging. In-vivo studies have observed that perfusion through such branches affects the (undesirable) progressive growth of the false lumen, preventing the (desirable) coagulation of blood in the false lumen and stabilization of the wall. It is therefore critical to understand pressure and flow in the false lumen. As non-invasive pressure measurements are challenging, FSI simulations prove to be a viable alternative and may present as a useful tool to provide guidance to the clinicians as to how and when to treat the dissected aorta. In this proof-of-concept study, we use an idealized anatomical model of the descending aorta to assess the impact of the presence of a side branch.
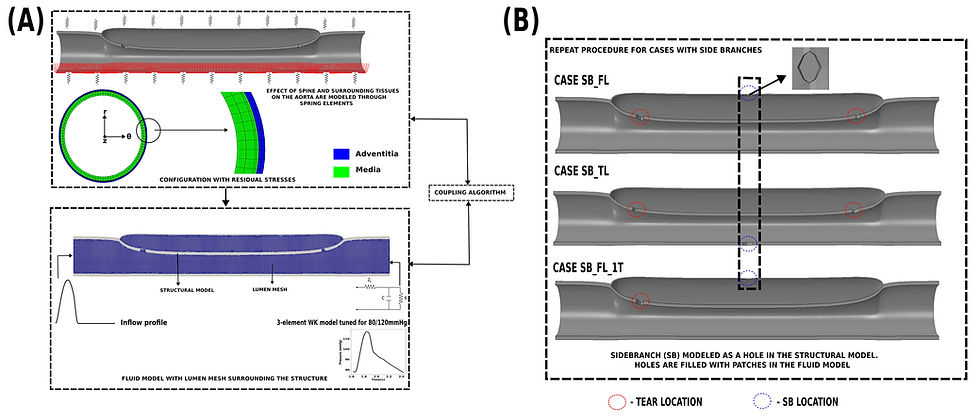
In our study, we start with a healthy descending aorta. While idealized in terms of geometry, the model includes all complexities of the aortic wall material. We then initiate the dissection, thereby obtaining any residual stress in the artery, and couple the fluid and solid equations using the in-house coupling tool CoCoNuT (https://pyfsi.github.io/coconut/) as shown in Fig 1(A). The reference model has two tears: one entry tear close to the inlet and a second re-entry tear near the outlet. Four cases are simulated (1) no side branch; (2) a side branch emanating from the false lumen (case SB_FL); (3) a side branch emanating from the false lumen, but with only one tear (case SB_FL_1T); (4) a side branch emanating from the true lumen (case SB_TL) as shown in Fig 1(B) with same total vascular resistance and state-of-the-art boundary conditions including surrounding tissue support and material anisotropy. The fluid and solid governing equations were solved on 96 and 16 cores of the AMD EPYC 7552 “Rome” processor, with each cardiac cycle taking roughly 24 hours.
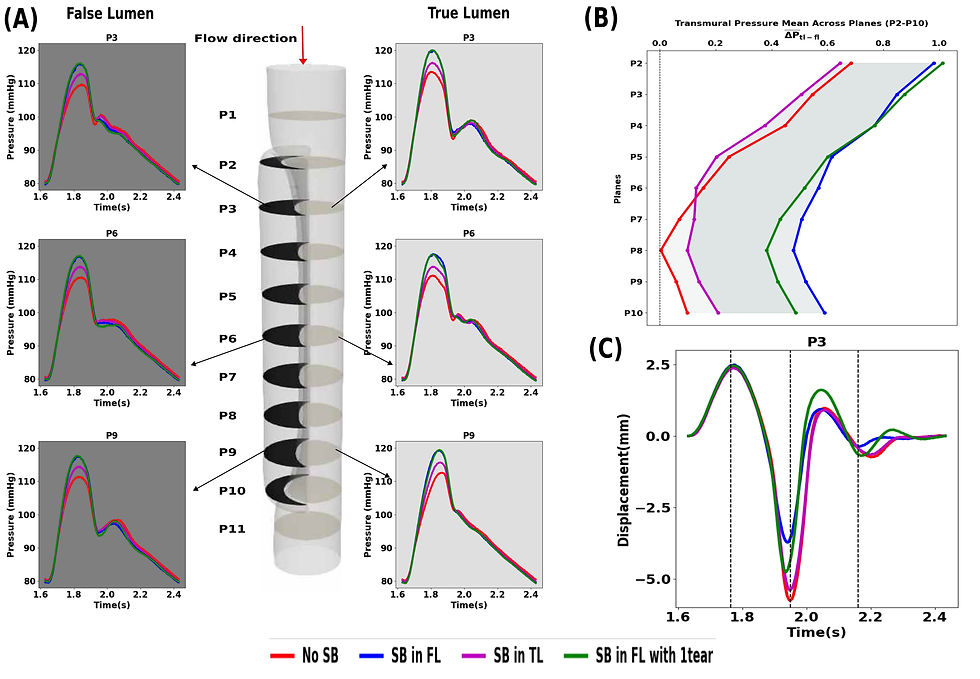
Intuitively, we expected the presence of a side branch to reduce pressure (think of poking holes in a pressurized elastic tube). However, our results after 3 cardiac cycles demonstrate that the side branch leads to higher pressures in both the true and false lumen during systole, with the highest increase in systolic pressure when it emanates from the false lumen (∼6 mmHg vs ∼3 mmHg when it emanates from the true lumen) (see Fig 2 (A)). We hypothesize that the pressure of the side branch reduces the functional buffer capacity of the aorta, most likely due to altered interactions between the true and false lumen. The average pressure difference between the true and false lumen (the transmural pressure) remains below 1 mmHg for all cases (Fig 2(B)), but with higher average differences across all planes when the side branch originates from the false lumen. Unlike most literature, our simulations yield very large and realistic displacements of the dissected membrane, up to 8mm in plane P3 for the case without side branches. The lowest displacement was found when the side branch originates from the false lumen, though still with displacement of ~ 5.5 mm (Fig 2(C)).
How did VSC contribute to our work?
Partitioned FSI simulations are computationally intensive, and access to the VSC infrastructure was invaluable and reduced computational times by weeks. Further, access to the Open OnDemand feature ensured that the entire process was within the same working environment, from case setup to post-processing.
Read the full publication in ScienceDirect here
🔍 Your Research Matters — Let’s Share It!
Have you used VSC’s computing power in your research? Did our infrastructure support your simulations, data analysis, or workflow?
We’d love to hear about it!
Take part in our #ShareYourSuccess campaign and show how VSC helped move your research forward. Whether it’s a publication, a project highlight, or a visual from your work, your story can inspire others.
🖥️ Be featured on our website and social media. Show the impact of your work. Help grow our research community
📬 Submit your story: https://www.vscentrum.be/sys




